Is it true that no two snowflakes are alike?
Despite all the paper snowflakes hanging in classroom windows, and shopping centers and malls looking much the same year after year, scientists say that the likelihood of two large natural snow crystals being identical is zero.
Below, find out what the researchers in the Library of Congress’ Science Reference Services had to say about just how unique snowflakes are.

A trillion trillion options means no two snowflakes are alike
The probability that two single ice crystals or flakes (a snow crystal or multiple snow crystals stuck together) will be exactly alike in molecular structure and in appearance, is infinitesimal.
Proving otherwise would not be easy, as each winter, about 1 septillion (1,000,000,000,000,000,000,000,000 — or a trillion trillion) snow crystals drop from the sky.
To go through all of the snow crystals produced every winter would be impossible, so we rely on cloud physicists, crystallographers, and meteorologists to study snow crystals and explain why there are no two snow crystals alike.
It starts with the water
First, we need to understand that not all water molecules are exactly alike. Generally speaking, water molecules have two hydrogen molecules with one 16O atom. However, not all water molecules will have this arrangement. Some water molecules will have an atom of deuterium in place of one of the hydrogen atoms, and some water molecules will have an atom of 18O.
Since the molecular makeup of snow crystals varies greatly from one to another, it follows that each snow crystal will be slightly different.
Furthermore, the unique and complex features of snow crystals are very much affected by unstable atmospheric conditions. Snow crystals are sensitive to temperature, and will change in shape and design as they fall from the cloud and are exposed to fluctuating temperatures. (See the video below for an illustrated explanation of this concept.) Therefore, to have two snow crystals or flakes with the same history of development is virtually impossible.

Back in 2007, new stories flourished that the old adage “No two snowflakes are alike,” might not be true.
What these stories were highlighting is that smaller crystals with simple shapes (e.g. hexagonal prisms) may look similar in appearance. The stories also reported that it is possible for snow crystals that have a small number (e.g. 10) of water molecules to be alike (a typical snow crystal contains 1018 water molecules).
As you can tell, depending upon how you define “alike” or “snow crystal,” you might find two snow crystals that are alike. However, scientific consensus still believes that it is very unlikely for two larger complex snow crystals to be identical in molecular structure and appearance.
Super-close up views of snowflakes
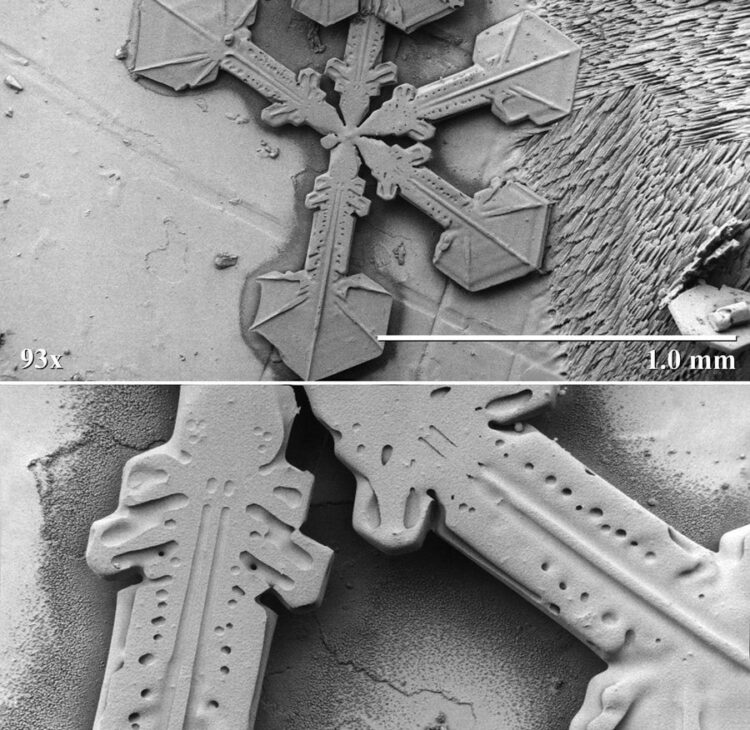
Most samples of snow crystals are observed at relatively moderate magnifications (30X-500X), however, the capabilities of the electron microscope allow observation of fine structures at over 100,000X.
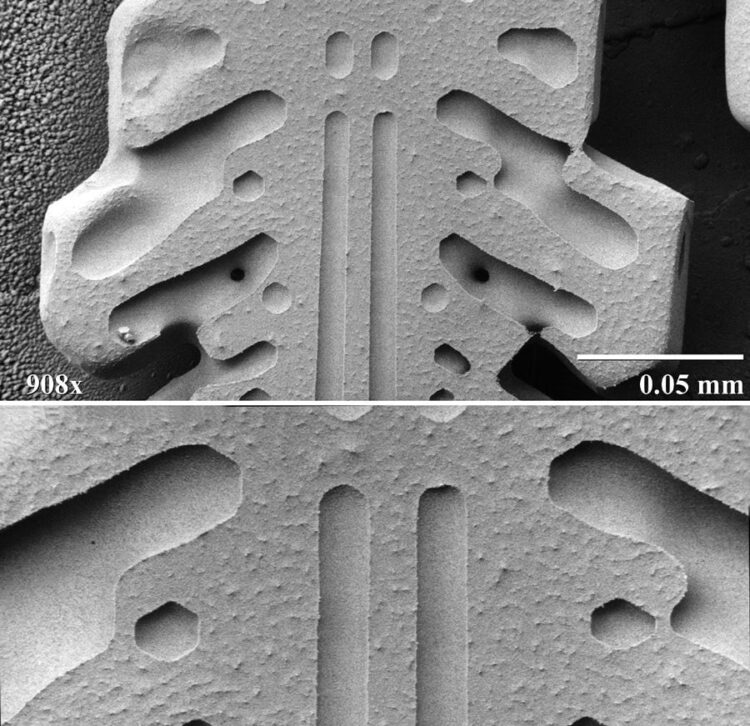
The black and white photographs shown in this article are from the Electron and Confocal Microscopy Laboratory, part of the US Department of Agriculture, and demonstrate a magnification series using a low-temperature scanning electron microscope (LT-SEM). Samples are frozen to temperatures below -170 degrees Centigrade, where they can be placed in a vacuum and observed for many hours with no structural changes.
About these images
These photographs show the extraordinary symmetry of snow crystals that’s apparent even at high magnification.
Image 1 upper: Single stellar snow crystal with sectorlike extensions, where one arm has been broken off during descent through the atmosphere. Magnified 100X.
Image 1 lower: Higher magnification view of two lower arms on the same snow crystal, on which a high degree of symmetry is noticeable. Magnified 300X.
Image 2 upper: Further magnified view of the left arm of the previous photo, in which parallel groove and pit structure shows a high degree of hexagonal structure and symmetry. Magnified 900X.
Image 2 lower: Smooth structure of groove and pit interiors as contrasted by asperities covered surfaces of etched crystal faces are visible at 1,800X.

The chemistry of snowflakes (video)
This video tracks the formation of snowflakes from their origins in bits of dust in clouds that become droplets of water falling to earth.
When the droplets cool, six crystal faces form because water molecules bond in hexagonal networks when they freeze. It explains that ice crystals grow fastest at the corners between the faces, fostering development of the six branches that exist in most snowflakes.
As snowflakes continue to develop, the branches can spread, grow long and pointy, or branch off into new arms. As each snowflake rises and falls through warmer and cooler air, it thus develops its own distinctive shape.
Video provided by the American Chemical Society







